What Variables and Units Are Used to Describe a Gas
The value for R depends on the units used for the other variables Table 2. Boyles law Chemist Robert Boyle stated that.

Gas Laws Cornell Doodle Notes Distance Learning Doodle Notes Teaching Chemistry Science Teaching Resources
The four gas variables are.

. Temperature pressure amount and volume of a gas are interdependent and many scientists have developed laws to describe the relationships among them. A nonflexible container is needed if the gas sample is going to have an internal pressure that is different from the external pressure. What statistical tests was used for the description in the Journal article Developing a systematic approach to obstetric emergencies.
This law explains how gases tend to expand volume increases when their temperature increases. -Take the shape and volume of its container. According to this theory gases are made up of tiny particles in random straight line motion.
Pressure P volume V the amount of gas nand temperature T. After completing this lesson you will be able to. The two variables have a direct relationship one increases so does the other and vic versa.
Rearrange the ideal gas law to isolate the unknown variable and preform any necessary unit conversions Solve for the unknown variable True or false. We will examine separately how the volume temperature and amount of gas each affect the pressure of an enclosed gas sample. Typically measured in moles.
The relationships between these variables are now known as the gas laws which describe our current knowledge about how gases behave on a macroscopic level. Pressure P volume V number of moles of gas n and temperature T. What are the 4 variables used to describe the characteristics of a gas.
Amount of gas n. In a Boyles law calculation any units of pressure and volume may be used as long as both the initial and final condition units match. Four variables are used to describe the condition of a gas.
Basically the ideal gas law gives the relationship between these above four different variables. Tap card to see definition. The Four Gas Law Variables.
All gases must be enclosed in a container that if there are openings can be sealed with no leaks. The three-dimensional space enclosed by the container walls is called volume. -Spontaneously fills its container.
Assuming that the questioner meant experiment where experince was written the volume of gas is the dependent variable and temperature is the independent variableThe French word for. Identify the variables that describe the sample. -Gases are easily compressible.
Variables used to describe a gas are pressure P 3 V 4 T and number of 5 n. Variables and Patterns - Ridley School District Overview. If we know 3 of the 4 variables we can use the IDEAL GAS LAW EQUATION to solve for the unknown.
What statistical tests was used for the description in the Journal article Developing a systematic approach to obstetric emergencies. When temperature increases gas molecules move faster because their kinetic energy increases and they make more impact with the container walls expanding the volume of the gas. J K-1 mol.
-Gases described by 4 variables. The pressure changes when the molecules change how often or how hard they hit. This activity is designed to look at what happens when you change 3 of the 4 variables.
PVnRT Where V volume of gas. Variables used for describing gases. In Section 103 Relationships among Pressure Temperature Volume and Amount you learned how the volume of a gas changes when its pressure temperature or amount is changed as.
Pressure is caused by molecules hitting the sides of a container or other objects. Describe steps in preparing for medication. The kinetic theory of gases also known as kinetic-molecular theory is a law that explains the behavior of a hypothetical ideal gas.
This is easy of course the units are mol. Click card to see definition. The SI value for R is.
The gas constant R. If you dont see any interesting for you use our search form on bottom. Describe a Medication Administration Record and its purpose.
You will usually be given the value for R if you need it. P pressure of the gas. To use the ideal gas law to describe the behavior of a gas.
R 8314 Pam3 K-1mol-1 8314 JK-1mol-1 8314 kPadm3 K-1mol-1 8314 kPaLK-1mol-1. If a flexible con-tainer is used the internal pressure and external pressure will always. Volume Temperature Pressure and Amount.
Volume L Temperature K Amount of Moles n Pressure atm kPa How is gas pressure produced. Gas law represents the unit in which it is measured and the abbreviation of the unit. ATwo variables are being held constant and there is a change in one that affects the other.
Temperature is related to the kinetic energy of the gas and is measured in Kelvin K. Therefore this problem can be reduced to one of the individual gas laws. List the name the symbol and a common unit for the four variables that are generally used to describe the characteristics of a gas.
Since the kinetic energy is mv 2 the same molecule will increase in velocity as temperature increases. The kinetic theory of gases is a topic that can explain many everyday observations. T temperature of the gas.
On this page you can read or download gas variables how are the variables that describe a gas related in PDF format. This law relates four different variables which are pressure volume no of moles or molecules and temperature. Values of the gas constant R for different units.
Natures and Properties if Gases. Lastly the constant in the equation shown below is R known as the the gas constant and it will always be the same. BOne variable is unknown and the other three are known.
9230 Mathematically Ideal gas law is expressed as. The temperature has to be in kelvins.

Factors Affecting Gas Pressure Read Chemistry Ck 12 Foundation

Classifying Chemical Reactions Using Chemistry Boom Cards For Distance Learning Free Science Worksheets Physical Science High School Distance Learning

The Scope Of Asme B31 4 Design Code Engineering Blog Coding Process Engineering Engineering

The Gas Laws Statements Formulae Solved Problems

Bart Simpson Controls And Variables With Answers Biology Worksheet Scientific Method Worksheet Scientific Method Worksheet Free

6 3 Combining The Gas Laws The Ideal Gas Equation And The General Gas Equation Chemistry Libretexts

Ideal Gas Law Calculator Pv Nrt

Using The Ideal Gas Law To Calculate A Change In Volume Worked Example Video Khan Academy

Quickstudy Physics Equations Answers Laminated Study Guide How To Study Physics Physics Classroom Physics Concepts

What Is Per Unit Price In Electricity Bill In 2021 Electricity Bill Cost Of Goods Sold The Unit

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Introductory Chemistry Lecture Lab

Pin On Learning For Your Brain Meats

The Gas Laws Statements Formulae Solved Problems

Concavity Ximera In 2022 Graphing Functions Inverse Functions Quotient Rule

Pin By Stefanie Mussman Pool On Science Ed Combined Gas Law Gas Law Gas Laws


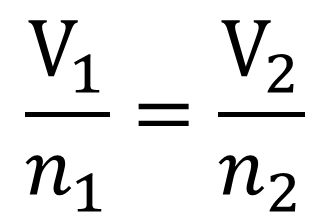
Comments
Post a Comment